Brief

When Covid-19 began its devastating spread around the globe in January 2020, healthcare companies were nothing short of miracle workers. Many rapidly pivoted, pushing aside multiyear development initiatives and other high-priority projects to focus on battling the pandemic. One company, an exemplar in the field of diagnostics, set out to produce a test that could detect SARS-CoV-2 and had a working product within three weeks. Less than two months later, the test was available for lab customers—a world-class time to market in diagnostics.
How did they do it? In a word: Agile. But it wasn’t always this way. Prior to the pandemic, the diagnostics company had managed innovation through a formal, multiyear stage-gate development process. Teams started with customer requirements gathering and product definition. Then, core teams worked in clearly defined swim lanes, with periodic executive input at major decision milestones. That linear and deliberate process was effective for products where the market evolution was gradual and the regulatory guidance was fairly clear. But it was no match for the uncertainty and urgency of the pandemic. The product development approach needed to incorporate a new cycle of learning, with weekly sprints and daily stand-ups.
As leadership teams across the healthcare industry know, this story is not an uncommon one. The pandemic ignited innovation, forcing new ways of working and faster, more frequent cross-functional decision making. Leaders became more intentional about where to apply standard procedures and where to shake things up with new ways of working.
But spur-of-the-moment agility is fragile. When the emergency fades, people eventually drift back to traditional, waterfall approaches to innovation. In reverting back to business as usual, they miss out on the chance to continue boosting their speed to market and accelerating ahead of the competition. And when the next crisis arises, the organization must undergo the painstaking process of shifting its operations and its people’s mindsets to Agile all over again. So why not maintain Agile approaches in typical times?
Some healthcare executives recognize that the pandemic has afforded them a rare opportunity to do just that. They are taking a few steps to build upon the foundation laid over the past 18 months and strategically apply Agile to other parts of the business, beyond those touched by the crisis. And they are finding that the payoff in results and performance can be huge.
Unlocking rapid innovation
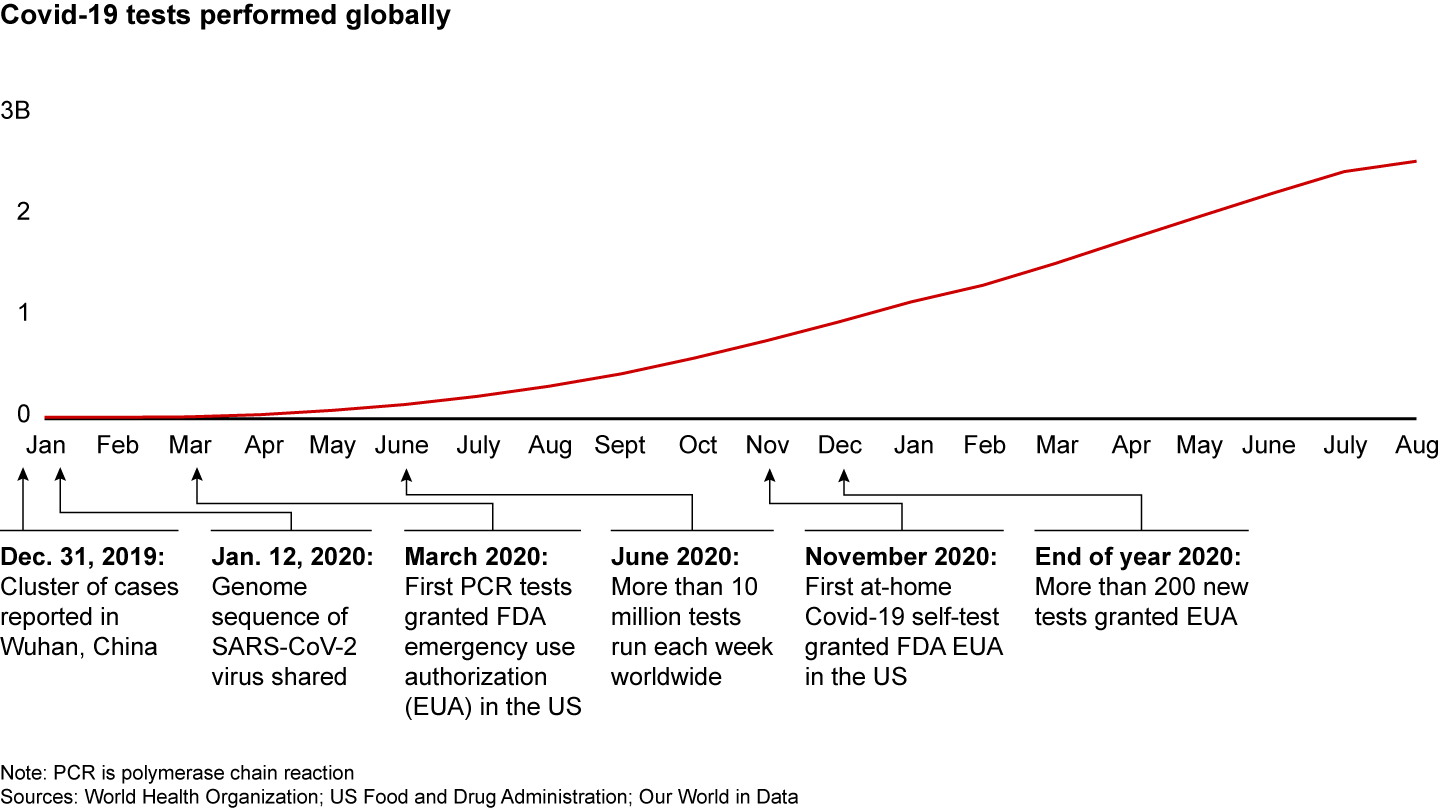
The tumultuous course of the pandemic makes a powerful case for Agile innovation in healthcare. By breaking free of conventional product development processes, companies helped quickly address a massive humanitarian crisis. As of August 2021, more than 2 billion Covid-19 tests had been administered, just a little more than a year and a half after the first case was reported (see Figure 1). And in the same time frame, 5 billion vaccine doses were administered.

Beyond the ability to rapidly address human need in the face of future crises, companies that sustain their newfound Agile ways of working can overcome long-standing hurdles to innovation. When considering innovation failures, senior healthcare executives cite ineffective cross-functional collaboration, ineffective collaboration with customers, and the failure to dedicate sufficient resources to scale projects as the most common causes. When done right, Agile addresses these problems.
And there’s never been a more important time to successfully innovate: Healthcare companies are under mounting pressure to improve quality and reduce costs. Digital insurgents like Amazon are disrupting traditional payers, providers, and pharma and medtech companies in their most profitable, competitive areas. To win in this increasingly turbulent and rapidly evolving landscape, healthcare companies will need to bring quality products to market even faster. By encouraging quick decision making on new product ideas, opening the lines of communication between the right teams, and elevating customer input, Agile primes the organization to swiftly respond and adapt to ever-changing customer needs and market conditions.

Doing Agile Right
Agile has the power to transform work—but only if it's implemented the right way.
Agile in action
What leadership teams do in the coming months could change the trajectory of their businesses. To stay on top coming out of the crisis and maintain speed, industry leaders are trying to embed the best of their new, Agile ways of working into the organization.
For the diagnostics company, it quickly became evident that the organization would need to preserve its new agility beyond its initial win with its first Covid-19 test. As Covid-19 continued to spread, testing needs endlessly evolved. Some lab customers wanted to enable patients to collect their own samples. Others, anxiously anticipating flu season, requested tests that could detect influenza in parallel with Covid-19. Large labs sought further automation and massive-scale testing equipment, while universities wanted fast-turnaround tests. And last winter, as variants became more prevalent, mutation resilience became a critical requirement.
To keep up with these continuously changing customer demands, the company realized it had to adapt parts of its multiyear product development and launch cycles. The organization accelerated timelines to meet the urgency of the pandemic through a few key changes to some of its internal processes and teams.
First, to rapidly update strategic priorities and match the lightning-fast cycle of the market, the diagnostic company adopted six- to eight-week strategy sprints. With new information about the virus arising on a near-daily basis, its strategy had to focus on helping product development address the most urgent market needs. Through short sprints, the diagnostics company quickly reviewed emerging customer needs and adjusted its product roadmap priorities and resources accordingly.
The process of defining products and their scope required a similarly dynamic approach. In the prepandemic multiyear development cycle, product definition was determined early on. During the pandemic, however, the company broke product programs down into modular elements. For instance, Agile teams identified workflow elements from one product that could be applied to another with slimmed-down bridging studies. The modularized product options defined a backlog of product innovation opportunities, which the team reviewed and reranked monthly.
The diagnostics company also needed to shift its customer feedback approach—there simply wasn’t time to conduct months-long customer surveys and wait for perfectly analyzed results. Instead, the company regularly took the pulse of the market by gleaning insights from informal customer discussions and bringing its commercial leadership team into biweekly product portfolio planning meetings.
Beyond customer feedback, the company introduced more frequent cross-functional collaboration throughout the product development process. Members from R&D, product, marketing, commercial, manufacturing, operations, and quality and regulatory teams attended the product portfolio meetings. By ensuring each meeting included the right set of attendees, the organization was able to quickly pinpoint issues, remove roadblocks, and make decisions.
Finally, the organization made other cultural and process changes in order to reduce time spent waiting on decisions, critical inputs, or funding. To maintain a sense of urgency, teams simplified lengthy business cases for product investment requests into a one-page template, cutting weeks of work for the product management team. They also created a checklist to easily communicate development progress and roadblocks to stakeholders, ensuring issues didn’t sit awaiting attention.
Through these critical changes, executives saw how the organization’s powerful burst of innovation could extend to other parts of the business beyond Covid-19 testing, enabling the company to mirror the speed of market change in the long run. Already, leaders have identified additional strategic opportunities for Agile ways of working, including new instruments and new diagnostic tests for other infectious diseases.
Pharmaceutical executives are also embracing the advantages of Agile as they try to speed time to market. Prior to the pandemic, one leading biopharma company noticed discrepancies in the performance of its clinical trial enrollment sites. It decided to launch a series of innovations around site start-up and enrollment support using Agile teams. The teams tested new prototypes with a panel of customers and site teams every two to three weeks. Within six weeks, the teams rolled out a new training program for clinical trial sites, and in the subsequent weeks, it followed up with additional innovations. As a result, the company accelerated the pace of enrollment, improved referral quality, and eventually, extended the practices to its entire profile of clinical trials. Today, for this pharma company and many others, the pandemic has reinforced and expanded the adoption of Agile practices, as organizations rolled out Covid-19 vaccines and therapeutics in record time.
Sustaining the power of Agile
Despite the industry’s dramatic successes during the pandemic, some healthcare executives remain reluctant to adopt Agile. They fear quality issues and slipshod trials, as well as a low return on investment. Their skepticism is not unfounded. In many cases, stage gates exist for a reason.
Indeed, some aspects of the product development process will need to keep a traditional planning cadence, depending on the regulatory, strategic, and market context. But winning companies will also tap into the speed and flexibility of Agile when market rate of change demands it, enabling fast decisions and rapid adaptation. Industry leaders understand there will always be “one-way door” decisions—those high-risk, difficult-to-undo decisions that still require a rigorous, methodical approach. But to sustain a dynamic environment, they will also encourage “two-way door” decisions—those low-risk, low-investment decisions that suit experimentation. They will find opportunities to test new ideas quickly and establish lightweight mechanisms to determine when to stop or continue certain initiatives. They will find a balance between keeping risks low and continuing to innovate quickly.
With the pandemic still fresh in their minds, executives have an invaluable opportunity to maintain the organization’s agility and strike the right balance. They can start by taking a few key steps.
Adopt short, frequent strategy sprints. Leading companies will improve their performance by setting bold, challenging objectives, and then establishing a regular, targeted sprint mechanism that matches the speed of market change. During these sprints, Agile teams assess market changes and lessons from previous sprints, then adjust their long-term forecast and strategic priorities where needed.
Encourage risk taking. Companies that excel at Agile exhibit a willingness to try new things and celebrate risk taking. They also question and change industry norms. When it became difficult for patients to visit clinical trial sites and doctors’ appointments early in the pandemic, one biopharma company quickly pivoted to keep clinical trials running, by adopting telehealth visits and sending medications to patients’ homes. Through these quick wins under the pressure of the crisis, the organization discovered the benefits of solving problems creatively and pushing industry boundaries.
Remove speed bumps. Most innovation teams spend just 15% to 20% of their time working on innovation—the rest is spent waiting on others for decisions. Companies that are geared for speed embrace frequent cross-functional operating mechanisms. These mechanisms are organized around a clear, specific outcome and focused on decisions and problem solving, not updates. For instance, when Ventec partnered with GM to ramp up ventilator production, the company made several changes to its traditional operating model. Teams used dynamic work planning to anticipate potential roadblocks two to four days in advance, established stand-up meetings twice daily, and reset expectations around details and control. As a result, GM-Ventec successfully delivered 30,000 ventilators to the US Department of Health and Human Services within 154 days, with the first batch shipped only one month after the introductory call between the companies.
Healthcare companies performed heroics during the pandemic—and it’s evident that their new ways of working can continue to create significant value for some time to come. The ability to make Agile innovation stick, even when the emergency passes, will differentiate industry leaders from the laggards.