Report

Резюме
- Although deal count and value declined in 2019, the medtech sector tends to fluctuate, so any single year’s tally does not necessarily indicate a larger trend.
- Corporates have a few structural advantages in medtech, and share of total acquisitions has been growing.
- Opportunities typically exist in niche, fragmented spaces with a path to category leadership, businesses providing an outsourced service to medtech manufacturers and consumer medtech.
- New or expanded regulations could impose greater costs and complexity on device makers over the next few years in Europe.
This article is part of Bain’s 2020 Global Healthcare Private Equity and Corporate M&A Report. Explore the contents of the report here or download the PDF to read the full report.
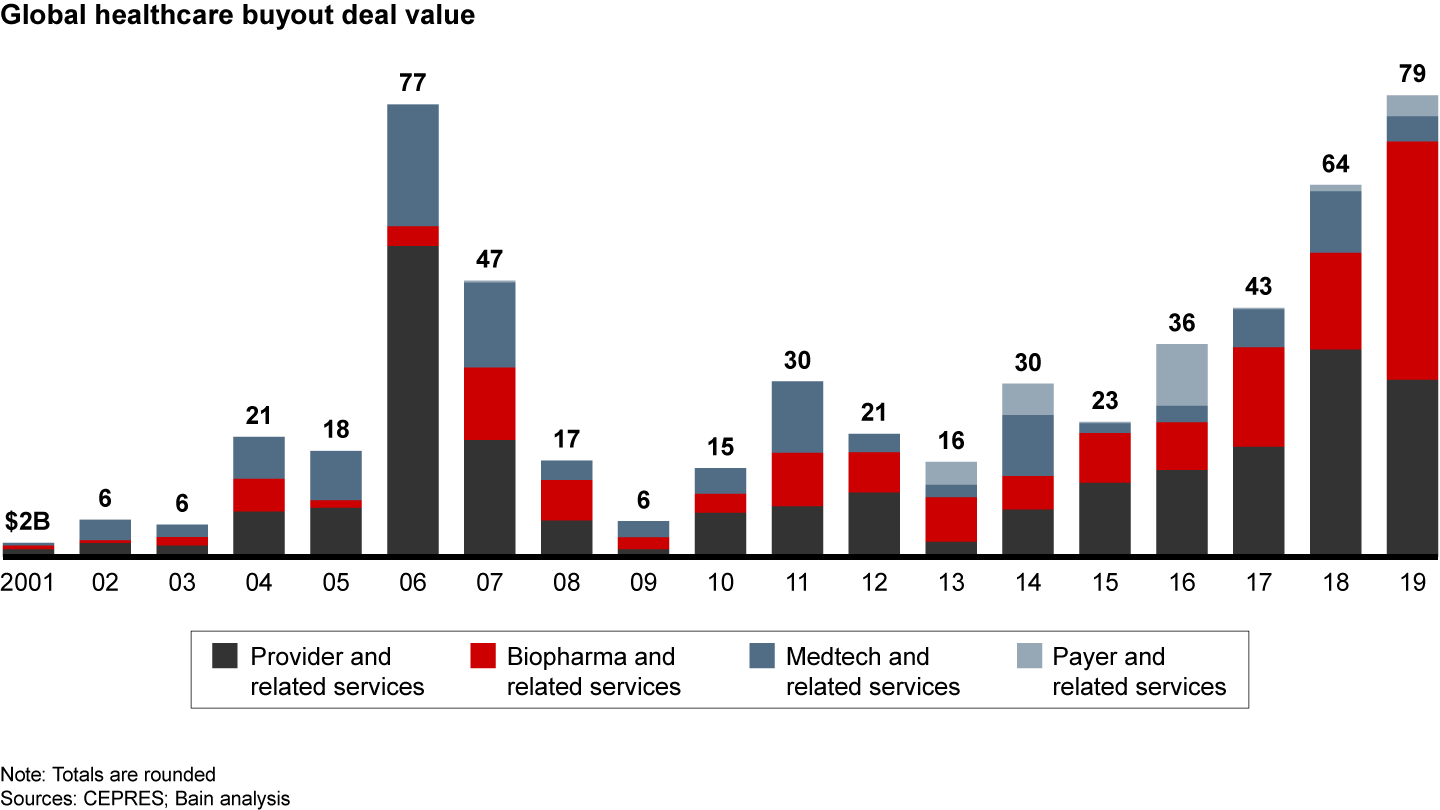

Investment in the medtech sector tends to fluctuate, so any single year’s tally does not necessarily indicate a larger trend. With that caution, a look back shows that medtech deal count dipped to 59 deals in 2019, following a record 67 deals in 2018.
Total disclosed deal value also dropped to $4.3 billion in 2019 compared with $10.5 billion in 2018, as private equity funds executed fewer deals greater than $1 billion. The year 2018 saw Platinum Equity acquire LifeScan for $2.1 billion along with four other deals worth between $1 billion and $2 billion.
From a regional perspective, most deals occurred outside North America. More local and regional incumbents in Europe and Asia-Pacific have attained scale and now attract interest, especially in the contract development and manufacturing spaces.
Corporates continued to make substantial investments in available assets during the year. Medtech direct investments can be capital intensive and require extensive manufacturing expertise compared with the service-based businesses that typically attract buyout funds. Corporates can underwrite higher values for medtech targets close to their core, often have deep expertise, and can extract value through scaling and improving sales and manufacturing operations as they fortify category leadership positions.
For example, Stryker announced an agreement to acquire Wright Medical Group, a medical device company focused on manufacturing and distributing extremities and biologics devices, valuing Wright at $5.4 billion, or six times the previous year’s sales. Johnson & Johnson acquired Auris Health, a developer of surgical robots, for $3.4 billion with additional contingent payments of up to $2.35 billion upon reaching certain milestones. Owning a standalone robotics company may not be as valuable to PE investors as it is for a corporate that can leverage the robotics platform to sell its broader device portfolio and offer a surgical system with both robotic surgery and medical implants.
Three investment themes lead the way
Despite strong corporate interest, medtech also continues to attract significant interest among PE investors. Many segments are riding favorable volume and demographic trends, and face manageable pricing pressure. Even lower-tech products can provide attractive economics through a consumable goods, razor-and-blades model. The sector provides relative stability and strong margins with mature categories, a competitive landscape and fairly sticky products dampening the chance of large-scale share shifts.
Three investment themes dominated medtech during the year:
- derivative services and IT plays;
- pursuit of category leadership; and
- consumer medtech and overlooked medtech categories.
1. Riding the trends through derivative services and IT plays
PE investors have been gravitating to the services segments that are not core capabilities for medtech companies, reflected by services making up 17% of medtech deals during the year. Service-centered segments don’t bear the reimbursement risk of healthcare-heavy assets. Indeed, healthcare-heavy medtech deals dropped from two-thirds to half of all deals in the sector, partly out of concerns over reimbursement changes, particularly in the US. The service investments allow sponsors to benefit from underlying fundamentals of the sector as well as the tailwinds of outsourcing trends as manufacturers continue to look for ways to cut cost amid price pressures.
Biopharma derivative plays provide a precedent, although medtech has primarily focused on outsourcing manufacturing, with less interest in outsourcing salesforce, development or clinical trial functions. (Most medtech regulatory approvals come through the 501(k) process, which is more lenient than the process for biopharma.)
Nordic Capital, for instance, bought Orchid Orthopedics Solutions, a medical device outsourcing service that does contract design and manufacturing, for roughly $1 billion. Nordic cited strong growth in the medical device market as well as Orchid’s ability to make its medical device manufacturer customers more competitive.
2. Pursuit of category leadership
As corporates look to rationalize their portfolios to attain category leadership or to implement regulatory mandated divestitures, investors have an opening to opportunistically carve out assets.
For example, Hillrom, a publicly listed US provider of medical technologies and related services, sold Aspen Surgical Products, a manufacturer of disposable medical products for operating rooms, to Audax Group for $170 million. Furthermore, as consumer medtech achieves substantial scale, PE sponsors could potentially capitalize as well, demonstrated by the $1.3 billion IPO of SmileDirectClub, an affordable direct-to-consumer teeth-straightening service.
Category leaders in small segments also merit capital from PE investors. Properly defining subsegments within medtech can uncover hidden category leaders. For example, Eurazeo Capital acquired DORC Dutch Ophthalmic Research Center, one of the few independent manufacturers of ophthalmic surgery instruments and equipment, for about $340 million. Another example was EQT’s investment in Clinical Innovations, a market-leading medical device company targeting labor and delivery and neonatal intensive care; EQT exited Clinical Innovations after only two years for a deal value of $525 million.
3. Consumer medtech and overlooked medtech categories
PE investors also play in specific categories that have a consumer focus or have been overlooked by broader medtech portfolios, often due to fewer sales-call point synergies. One such deal was Clayton Dubilier & Rice’s carve-out of Cynosure, a company that manufactures medical aesthetic treatment systems for a variety of healthcare practitioners, from Hologic. Cynosure’s aesthetics business had little call point overlap with Hologic’s women’s health products, making it logical to carve out of the business.
Regardless of investment theme, medtech investors have several routes to reap good returns. One is to consolidate smaller firms within fragmented categories in order to create a category leader. Another is to implement commercial excellence or cost-reduction programs, as owners of assets have historically have been less of a strategic focus for their corporate parents. For capital-intensive purchases, owners will need to lean in more on operations, but they do have a viable playbook for creating value.
How expanded regulations could play out
The year saw government agencies clarify many regulations on the books, which made investors more comfortable in underwriting assets. China and India implemented new medical device regulations in 2017, which established more structured systems. Despite this greater clarity, medtech in Asia-Pacific fell to 10 deals in 2019, compared with 18 in 2018.
In Europe, deal count remained steady at 24 in 2019, compared with 23 in 2018. The Medical Devices Regulation (MDR) and In Vitro Diagnostic Medical Devices Regulation (IVDR) increased transparency for investors in the region. MDR comes into force in May 2020. IVDR comes into force in May 2022. Legacy devices must comply with new standards at the latest by 2025.
The implications for manufacturers include higher operating costs over the next few years due to investments in data generation and regulatory advisory, the risk of products being taken off the market due to a backlog of applications, and potentially more complexity in executing clinical trials.
A wealth of favorable trends for the near future
Looking out the next few years, we expect investors will continue to ride the high profit margins and recession-resistant nature of the sector as well as favorable volume and demographic trends:
- positive demographics, especially in certain subsegments such as orthopedics;
- manageable pricing pressure, more from the sophistication of provider procurement than from government intervention;
- mature categories and sticky products;
- potential for consolidation within categories;
- attractive economics from a razor-and-blades consumable goods model for many lower-tech products;
- large cost-take-out opportunities for undermanaged companies;
- potential for corporate exits; and
- less regulatory risk in the US compared with sectors such as provider.
Corporates will present stiff competition for assets, so PE investors probably will focus more on efficiency plays with contract manufacturing organizations and other firms that improve operations through digital and IT solutions. Going forward we expect investors to continue to focus on derivative plays and healthcare-light assets.
This article is part of Bain’s 2020 Global Healthcare Private Equity and Corporate M&A Report. Explore the contents of the report here or download the PDF to read the full report.