Report

Резюме
- European total disclosed value surged about 40% in 2018, to $17.8 billion, while deal count remained steady at 73.
- Funds were particularly active in the provider sector, despite a steep drop from 2017, and in medtech.
- Several pharmaceutical megadeals drove the increase in values.
- Regulatory and political trends, ranging from Germany’s regulatory window to Brexit, could potentially generate investment opportunities.
This article is part of Bain’s 2019 Global Healthcare Private Equity and Corporate M&A Report. Explore the contents of the report here or download the PDF to read the full report.
-
Click to Expand: Regional Overview
Global healthcare PE activity set another record in 2018, with 316 announced deals valued at $63.1 billion—a marked increase over $42.6 billion across 265 deals in 2017. Activity and disclosed value rose in every region, an increase that was especially pronounced in Asia-Pacific, where the volume of announced deals grew by 44%.
The rise in values stemmed primarily from more large buyouts, as eight deals in 2018 were valued at greater than $2 billion each vs. just four in 2017. Across regions, the provider sector again was the most active, accounting for more than half of total deal activity and disclosed value. Biopharma posted the second-largest share, accounting for roughly one-quarter of volume and value globally.
Similar to prior years, the countries outside North America, Europe and the Asia-Pacific region saw minimal healthcare PE deal activity in 2018. There were six buyouts, two more than 2017, with no deal values disclosed. Of those six buyouts, four occurred in the provider sector, and three of those had Brazilian targets. This was a departure from 2016 and 2017 activity, which each saw just one deal in the provider sector and one Brazilian target.
European deal activity remained robust in 2018, as deal count nudged to 73, up from 70 in 2017. Average European deal size surged in 2018, however, as disclosed value reached a record $17.8 billion. This bump in value came mostly from two large biopharma deals, Recordati and Zentiva, cumulatively worth $9.8 billion, which was $3.8 billion more than the top two European deals from 2017 (see Figures 5 and 6).
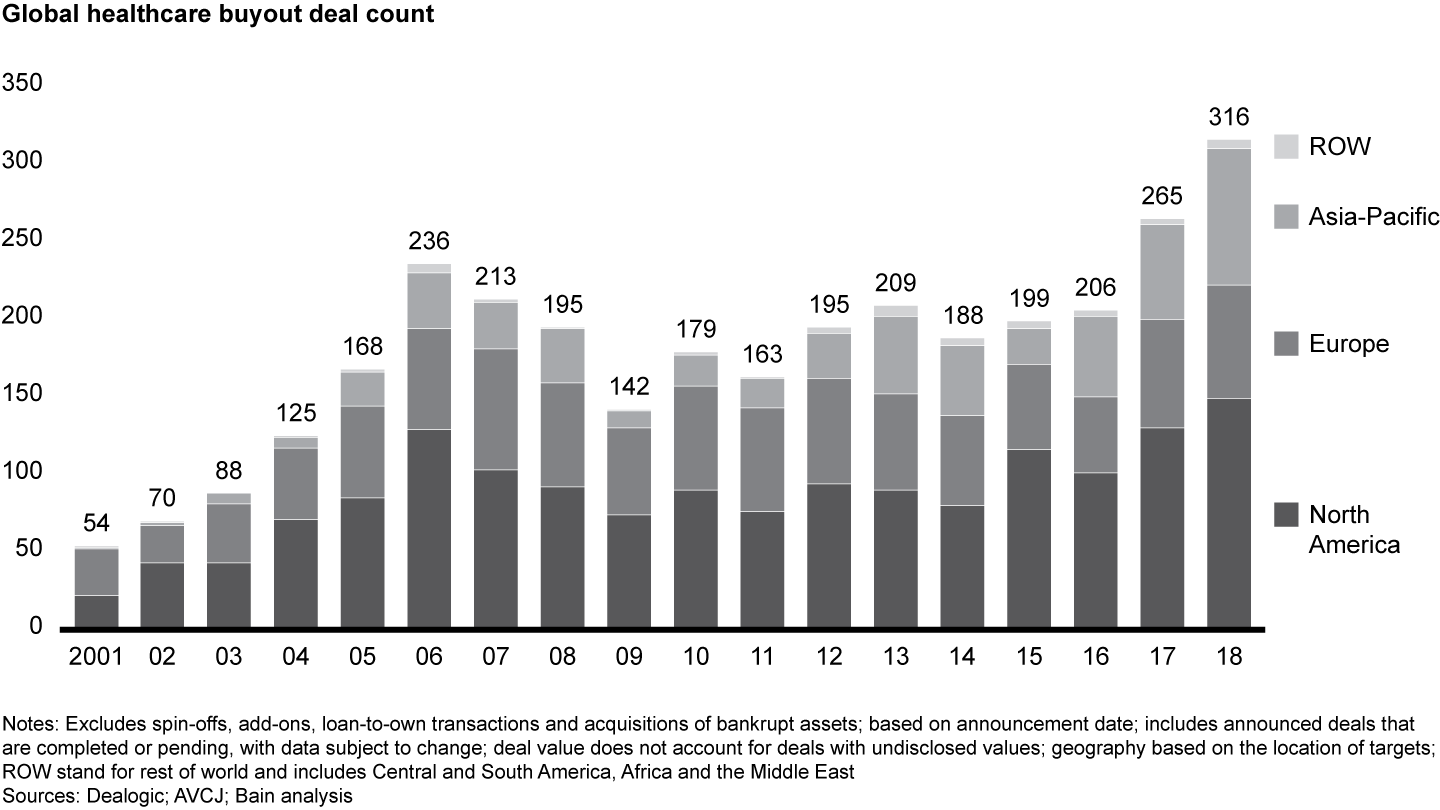


As Europe’s economy slows, the perceived safety of the healthcare industry has continued to attract investor interest. Total disclosed deal value in the region surged about 40%, to $17.8 billion, in 2018, up from $12.8 billion in 2017. Large deals in the biopharma and medtech sectors accounted for four of the top five European healthcare PE deals, all greater than $1 billion.
Deal count in 2018 stayed relatively flat at 73, compared with 70 in 2017. With average deal size increasing, however, investors continue to band together through financial sponsor consortiums and corporate partnering so that they can write larger checks. Four of the top 10 deals in Europe involved consortiums or corporate partners, including the largest deal of the year for the region: CVC Capital Partners, PSP Investments and the StepStone Group’s acquisition of Italian pharmaceutical manufacturer Recordati for $7.4 billion.
Retail health played a prominent role during the year. Investors have been expanding buy-and-build strategies in retail health by moving into more countries. That tack motivated several deals in veterinary and dental services. For example, BC Partners acquired VetPartners for $900 million in the UK, a market that has seen considerable consolidation in recent years, and Nordic Capital’s acquisition of three dental groups in Germany, Switzerland and the Netherlands, along with one large dental laboratory in Germany, represents the brisk consolidation in these countries that we noted in last year’s report.
There is a limit, however, to the extent of cross-border growth by retail health platforms, mainly because of reimbursement system and language constraints. These limitations apply mostly to provider and HCIT companies involved in procurement, payments or administration, because healthcare delivery and regulations differ from country to country. Moreover, healthcare companies can tap only a few types of cross-border synergies, such as efficiencies in purchasing supplies, regulatory risk diversification and financial scale. Knowing where the geographic, systemic and scale barriers apply helps investors execute a sound expansion strategy.
Bain Partner Franz-Robert Klingan discusses how investors can succeed despite high demand for healthcare assets in Europe.
Investors also continued to show a willingness to take on reimbursement risk by investing in healthcare-heavy assets. Funds have stepped up investments in providers and biopharma as they develop more sophisticated ways to underwrite this risk and dial back the level of uncertainty. To be sure, part of this shift stems from necessity and the quest for strong returns in a historically competitive market, but it’s also true that investors have equipped themselves to appropriately incorporate higher-risk assets into large and growing portfolios. More than one-third of European investments in 2018 had some direct reimbursement exposure, including Advent International’s acquisition of Zentiva, a collection of European generics businesses from Sanofi, for $2.4 billion.
Turning to biopharma, investors showed a willingness to make bigger bets. They looked for companies with regional category leadership in order to establish supply security and negotiating leverage with government payers. Although deal activity stayed flat, biopharma deals accounted for $11.0 billion of the region’s $17.8 billion total, led by the Recordati buyout. A renewed investment cycle across prescription generics and over-the-counter (OTC) drugs has also begun. Despite political pressures on pricing, investors have a solid understanding of pricing dynamics in Europe. Opportunities abound to acquire noncore assets from large corporates looking to rationalize their portfolios in order to fund their own drug pipelines.
While a majority of deals still get sponsored by European investment vehicles, Chinese investors also are looking for opportunities to gain experiences or capabilities that they can apply back in China. Inner Mongolia Furui Medical Science partnered with Astorg Partners to acquire a minority stake in Echosens, a manufacturer of liver diagnostic equipment, for $200 million, as one way to accelerate growth in North America and China. And European funds have shown interest in North American assets. For example, Swedish firm Investor AB acquired a majority stake in Sarnova Holdings for $500 million through its long-hold vehicle Patricia Industries.
More broadly, other regulatory and political trends represent opportunities in the short term, even if they raise uncertainty in the longer term.
- Pending German reforms: Germany, the largest European healthcare market, is mulling a series of reforms, including tariffs for hospital finance and regulatory requirements for running retail health chains. Other potential reforms address providers, potentially creating clarity and a stable regulatory outlook.
- Brexit: While investors gained a better view of Brexit’s potential impact on the EU healthcare landscape, negotiations over Great Britain’s EU withdrawal continue. Brexit could lead to a shortage of healthcare workers, presenting both opportunities and risks throughout the system.
- Medical Device Regulation (MDR) reform: New regulations will increase the cost of medical device research and development (R&D) and manufacturing by raising the standards for clinical data and approvals; however, they also will provide investors with increased certainty through more standardized risk classifications and through improved transparency. Increased certainty should give investors better insight into the risks and opportunities related to MDR when underwriting assets in this space.
- New general data protection regulations (GDPR): Implemented in May 2018, GDPR will likely add costs for companies, possibly triggering a need for additional capital and deal activity.
Demand for European healthcare assets should hold steady in 2019 as investors continue to seek stability in their portfolio and adjust to the changing regulatory landscape. We expect an uptick in certain countries, such as the UK, and certain sectors, such as HCIT, as investors gain further confidence about dealing with new regulations. Pharma will also continue to attract interest as investors seek regional category leaders and actively work on their corresponding portfolio assets.
China and other Asia-Pacific investors likely will increase their activity in Europe to continue learning about segments, such as home care, that will likely take on more relevance and to expand market access in home markets. Finally, we expect European funds to further raise their activity in North American investments as North American healthcare assets outperform European healthcare assets through recessions, according to CEPRES analysis (see “2019 and beyond: Uncertainty in many markets, but healthcare private equity may be an oasis of relative calm”).