レポート
 }
}
概要
- The year 2019 saw unprecedented regulatory scrutiny of M&A transactions.
- In a tech-enabled world, deal scrutiny is expanding to include issues such as data ownership and access to critical technology, often prompted by national interests, as well as the impact on future competition.
- The time, complexity and the resources to get deals over the regulatory hurdle are rising—it will take longer than you think and cost more than you expect.
- This all places greater demands on preparing for regulator consultations and broader stakeholder communications during the deal diligence and negotiation phases.
This article is Section 2.1 of Bain's 2020 Global Corporate M&A Report. Explore our latest annual M&A report here.
Scale deals are getting harder to clear
Many industries are reaching their natural limits on consolidation, a nagging reality that makes it harder to get deals approved. Consider T-Mobile and Sprint’s $59.6 billion merger in the US. After two unsuccessful bids, it was finally approved by the federal regulatory authorities, but the deal still faces objections from multiple states. In Asia-Pacific, regulators blocked the $6.2 billion merger of Vodafone Group’s Australian business with TPG Telecom. In Europe, the Competition and Markets Authority (CMA) halted the $10 billion Sainsbury’s-Asda merger.
European regulators have also been hawkish on pan-European deals. In February 2019, the European Commission (EC) blocked the merger of Siemens Mobility and Alstom's rail transport business. The companies failed to address the EC’s concerns regarding competition in the signaling and high-speed rolling stock markets. Alstom CEO Henri Poupart-Lafarge said, “If I have one regret, it is using that phrase ‘European champion.’” The EC also blocked a joint venture between Germany’s Thyssenkrupp and India’s Tata Steel (Europe) in June 2019, which would have created Europe’s second-largest steel producer.
For companies undertaking scale deals, there are proven ways to prepare for navigating regulatory consultations.
Let’s look at the proposed merger between the No. 3 and No. 4 telecommunications players in a European country. The two companies were offering mobile services in the same market, with a combined market share of 25%. The EC had a past record of requiring remedies or even blocking such in-country telecom deals. Over the previous five years, Europe saw two such mobile-mobile scale deals rejected or withdrawn, and five were approved with remedies, which, over time, progressed in their severity.
Knowing this history, the acquirer still decided to go ahead but to prepare rigorously and perform vast and in-depth analysis on issues such as:
- local and European market and competitor analysis, including both core mobile and adjacent markets;
- infrastructure investments made by players and the outlook for future developments;
- business rationale for the deal and the expected benefits, in particular to customers;
- risks for the two companies in the event that they did not merge and the effect on competition; and
- merger efficiencies (synergies) and their specificity and verifiability.
The comprehensive analysis addressed the regulator’s concerns by showing how the deal would be a win-win for the merging companies and customers as it would both improve services and increase competition. The robust responses prepared to address all political and competitive arguments against the deal were strong enough to allow the acquirer to ask for and receive unconditional approval from the EC.
The time, expense and required remedies to get scale deals approved is rising. Increased regulatory scrutiny—enabled by digital technologies such as e-discovery, which can scan hundreds of thousands of documents on company IT systems and devices—raises the bar on deal evaluation, due diligence and the depth of preparation needed for regulatory filings and engagement.
Companies need to be ready for a longer time to close, including the timing implications on value capture and, more importantly, the people costs. A lengthy time to close can wear out the most motivated leadership teams, and as regulators ask for more internal data, it can also drain other resources. Assessing regulatory risks at the diligence stage and preparing for remedy negotiation ahead of time bring great value.
Scrutiny on nontraditional grounds is rising
The rising tide of populism and widespread angst around economic and political well-being are raising questions about the role of large corporations and their influence over day-to-day life. The resulting political pressure has led to some fundamental rethinking about antitrust laws and national security mandates in approving deals. As such, regulatory oversight is evolving beyond issues of market concentration to include consumer data and privacy, national interest and security, and future competition. Some sectors, such as technology, aerospace and defense, telecommunications, and healthcare, lend themselves to more scrutiny on nontraditional grounds than others do.
There are several recent instances of blocked deals and even rescindments on these grounds. In 2019, the Committee on Foreign Investment in the United States (CFIUS) required Beijing Kunlun Tech to sell Grindr, a dating app purchased over 2016 to 2018. Some believe that the request came after scrutinizing app developers over the safety of the personal information of millions of Americans. (CFIUS did not disclose the reasons.)
Around the same time, CFIUS demanded that Chinese digital healthcare company iCarbonX divest its interests in PatientsLikeMe and HealthTell, two US companies that collect health data. While the reason for CFIUS’s decision in this particular deal is unknown, it was speculated that the regulatory body had concerns that the private health data of American citizens would be exposed to the Chinese owner.
In September 2019, the US Department of the Treasury proposed regulations to broaden the CFIUS’s oversight to smaller foreign investments that involve a critical technology, critical infrastructure or sensitive personal data of US citizens.
Recently, regulators have increased scrutiny on what are being termed “killer acquisitions.” In these deals, a large company acquires innovative targets only to discontinue the development of the targets’ innovative projects to prevent future competition. Indeed, acquisitions of start-ups by larger incumbents could eliminate future competitive threats, increasing the incumbents’ overall market power in a way that would not be captured with traditional market share analysis. These acquisitions often go undetected due to low deal value, scale or consumer impact.
Amazon’s attempt to take a minority stake in Deliveroo, an online food delivery business, through a financing round with several other investors (totaling $575 million) is under inquiry by the CMA after German authorities had already cleared the deal.
This type of scrutiny is not only limited to tech companies. Mexican officials blocked Walmart’s deal to acquire Cornershop, a small grocery delivery business. The deal was blocked on the grounds of unfair access. Walmart could not guarantee a level playing field for rival retailers whose customers use the application to order groceries and other goods.
Even in pharma, deals in which a big company acquires a nascent rival and halts its drug development are coming under scrutiny. Questcor (currently Mallinckrodt ARD) had a US monopoly on adrenocorticotropic hormone drugs with its Acthar formulation. It acquired the US rights to a potentially competing drug, Synacthen Depot, in 2013, and later stopped its development. Acthar’s vial price was raised from $40 in 2001 to $34,000 in 2015, while outside the US, Synacthen Depot was available at a fraction of Acthar’s price, according to the complaint. To settle the complaint, the company agreed to grant a license to develop Synacthen Depot and pay a $100 million fine to the Federal Trade Commission (FTC) for violating antitrust laws (by preventing another bidder from developing and launching the drug in the US).
Lately, the FTC chairman has gone so far as to say that he’s ready to break up major technology platforms if necessary by undoing their past mergers. For many companies, this is new territory to navigate (see Figure 2.1).
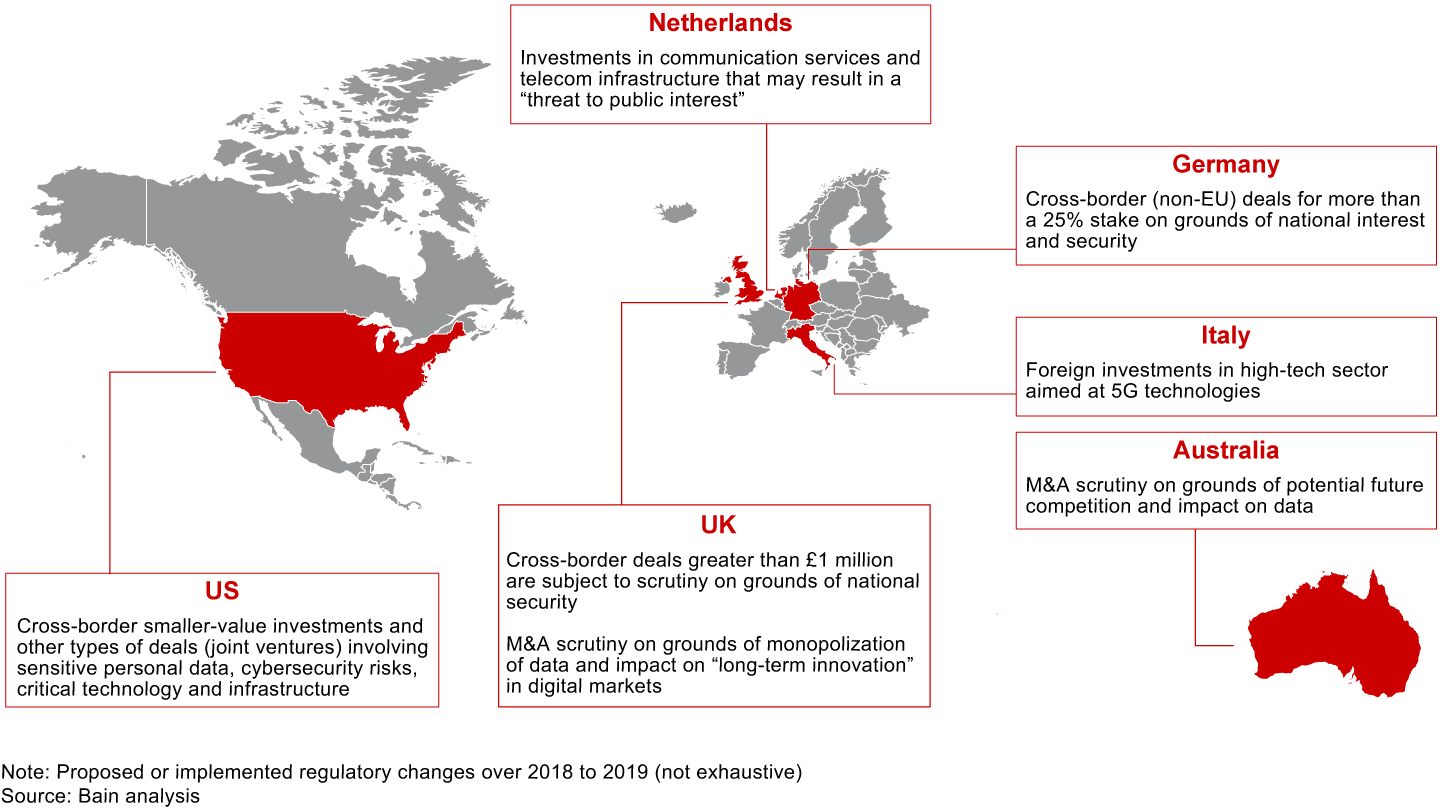

For dealmakers, scale and market power are no longer the only considerations to get regulatory approval. Addressing the evolving regulatory discussions and stakeholder communications will require greater thought and preparation, but doing so can pay off by reducing the friction and costs required to get a deal over the regulatory hurdle.
This article is part of Bain’s 2020 Global Corporate M&A Report. Explore the contents of the report here or download the PDF to read the full report.