Rapport

En Bref
- Biopharma buyouts maintained near-record levels in 2018, totaling $16.5 billion across 79 deals, compared with $17.0 billion across 62 deals in 2017.
- Despite political and media scrutiny of drug pricing, investor interest in biopharma remains strong globally. Larger buyout firms had ample appetites for pharma platforms. Some funds even established investment vehicles focused on life sciences, including those that can handle assets with clinical risk.
- Deal activity in outsourced pharma services continues to be strong, with a sharp focus on niche contract organizations.
This article is part of Bain’s 2019 Global Healthcare Private Equity and Corporate M&A Report. Explore the contents of the report here or download the PDF to read the full report.
Biopharma and related services came in as the second-largest sector in global healthcare private equity by volume during 2018, with 79 deals accounting for one-quarter of total deal count. At $16.5 billion, disclosed value of biopharma deals came close to the $17.0 billion in 2017 on an absolute basis (see Figure 7). As a share of total disclosed value, however, biopharma dropped to about 25% from about 40% in the previous year. Increased interest in biopharma platforms spurred volume, and PE investors started taking on clinical and reimbursement risk in the sector.
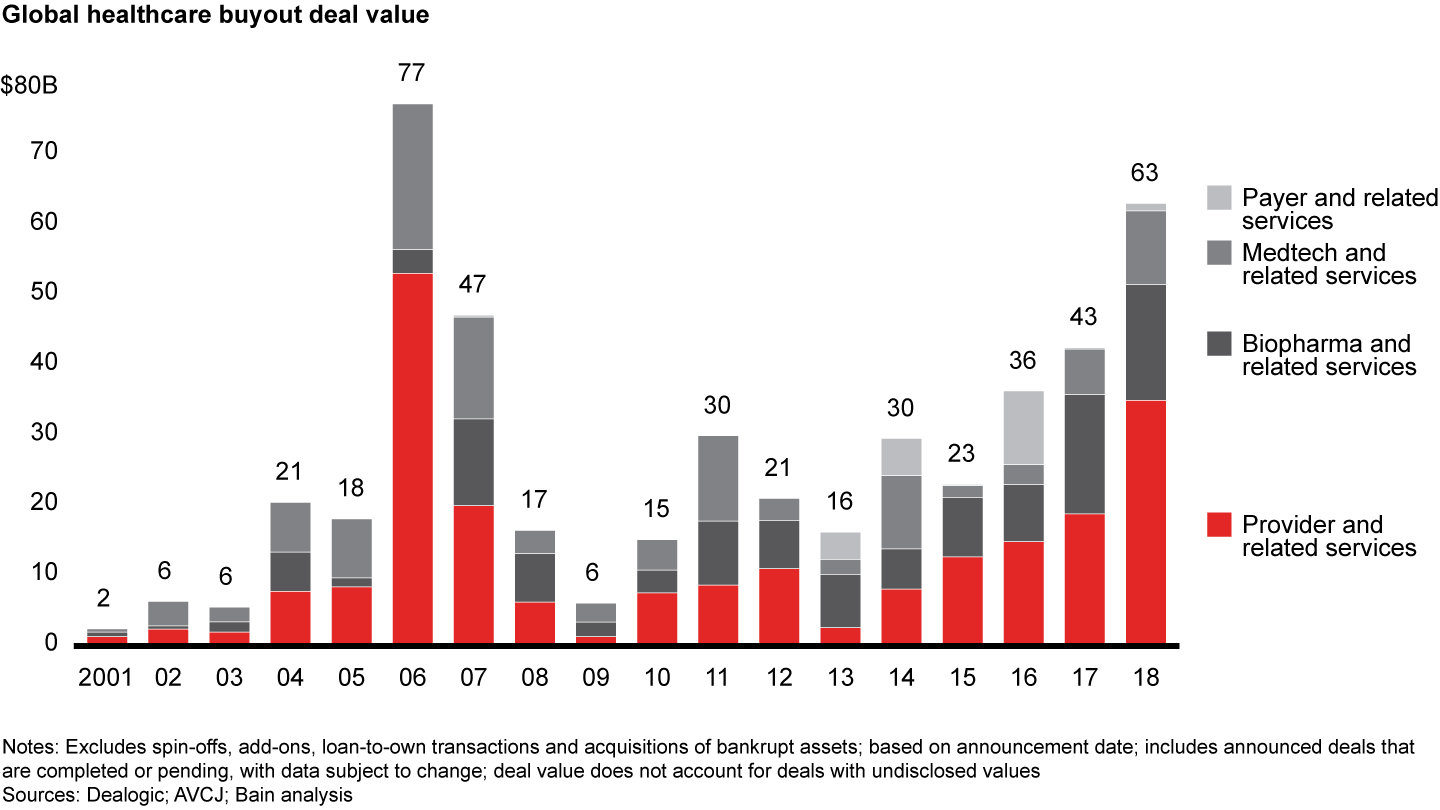

Although the biopharma industry continues to navigate a tough pricing and regulatory environment, investors are still drawn to the sector’s shareholder returns and high margins. These public shareholder returns historically have been driven by revenue and margin expansion rather than multiple expansion. Because of biopharma’s durable generics volume and lengthy intellectual property protection for novel therapies, investors perceive it to be the segment most resistant to economic downturns.
These traits translated to strong PE activity during 2018, with a total disclosed value of $16.5 billion across 79 deals, compared with a record $17.0 billion across 62 deals in 2017. Deal value was boosted by several deals greater than $500 million, including CVC, PSP Investments and StepStone Group’s $7.4 billion acquisition of Italian pharmaceutical company Recordati and Advent’s $2.4 billion acquisition of generics manufacturer Zentiva. Increased deal activity in Asia-Pacific, particularly for earlier-stage investments, helped drive the rise in global deal count.
Investors in the US continued to be more interested in novel and originator pharmaceuticals, which have historically enjoyed strong price positions and margins; however, the industry has faced some headwinds in the US recently. Political and media pressure on drug prices during the year led some major pharmaceutical companies to freezing scheduled price hikes temporarily in July. Despite this increased scrutiny, dozens of drugmakers still executed price increases exceeding inflation on hundreds of drugs in January 2019.
Payer consolidation with PBMs in the US, such as Cigna’s acquisition of Express Scripts, may also result in increased pricing pressure as health insurers have announced their intention to lower prices. The cost to innovate is increasing, and innovation continues to come largely from outside Big Pharma, creating even more pressure to source the right deals earlier in development. These developments highlight why funds will want to make thoughtful investments in category-leading drugmakers as well as in service providers or suppliers that help drugmakers bring new molecular entities to market cost effectively.
In Europe, investors continued to be more focused on manufacturers of mature branded and generic pharmaceuticals, as evidenced by the Recordati and Zentiva deals. The CVC-led consortium was able to acquire a controlling stake in Recordati at a discount to its public market valuation as part of a handover from the founding family. New ownership could lead a portfolio refresh through follow-on M&A in order to accelerate growth.
Investors have found value in these types of stable, regionally scaled assets in Europe, where parallel country-specific healthcare systems, supply reliability issues provoked by overly aggressive tendering and repeat compliance issues with non-EU supplies have moderated the continued pressure on generic prices to a manageable level. Investors also turned to the public markets to acquire drug manufacturers, as evidenced by several large deals, including EQT announcing it will take Karo Pharmaceuticals private for $963 million (including net debt).
Funds showed an increased appetite for pharma platform investments during 2018. Drugmaker deal count rose from 33 in 2017 to 38 in 2018, and disclosed deal value increased from $8.2 billion to $14.7 billion. Two themes emerged in these acquisitions: earlier investments in clinical-stage assets; and large checks written by large-cap funds. Part of the rationale is that buyout firms can run efficient R&D programs unburdened by the established Big Pharma paradigm and can be quick to move on from underperforming programs.
One of the biggest investments last year was Bain Capital’s acquisition of 10 development-stage neuroscience programs from Pfizer for $350 million, creating a novel branded pharma company, Cerevel Therapeutics. Pfizer will continue to own 25% of the new company, with senior program management staying in place. This represents the continuation of a clinical-stage pharma platform strategy first employed in 2017, when Bain Capital Life Sciences invested in a $103 million Series A round for SpringWorks, a collection of four clinical-stage rare disease programs spun out of Pfizer. Notably, the price for the Cerevel Therapeutics deal exceeded a typical investment by a venture capital firm or club. Through this deal, Bain Capital has elevated itself into a league of its own in terms of clinical-stage carve-outs.
As larger buyout firms become more interested in developing biopharma platforms, a few have launched their own investment vehicles focused on life sciences. Bain Capital raised a $720 million life sciences fund in 2017, with the intent of making investments between $30 million and $70 million in both private and public medical device, specialty pharmaceutical and biotech companies, including several deals in 2017. Blackstone acquired Clarus, a life sciences investment firm focused on growth-stage biopharma investments, in 2018 and renamed the new division Blackstone Life Sciences. The division will focus on providing capital to underfunded biopharma programs to develop and commercialize new drugs. TPG established TPG Biotech, a venture capital fund focused on life sciences companies, in 2002; it now has more than $550 million in assets under management.
Investors also continued to show strong interest in biopharma service providers and HCIT solutions that support drugmakers in developing, manufacturing and commercializing new therapies, as biopharma services deals held steady from 2017, accounting for 27 of the 79 deals in 2018. Investors are widening their focus beyond traditional, broad contract organizations, known collectively as CXOs, however, to target niche CXOs that operate in nascent or emerging service lines or on firms that serve specific populations.
Niche CXOs attract interest partly because of the consolidation and maturation of the CXO industry and partly due to the rise of specialty drugs that have a different set of needs. For example, Linden Capital Partners acquired Evolution Research Group, a research site service company focused on special populations for central nervous system studies. And Genstar Capital acquired CRF Health in July, combining the provider of electronic clinical outcome assessment (eCOA) and electronic consent (eConsent) solutions with its portfolio company, Bracket, a clinical trials software provider. The new entity will provide a broader suite of services to manage clinical trials effectively and improve the patient experience. While there are limitations to scaling these investments in their original niches, they can provide investors with a foothold to develop a platform of adjacent capabilities. We expect investors to continue to seek niche CXO opportunities along the drug development value chain.
There was also continued investor interest in contract development and manufacturing organizations (CDMOs), specifically, which provide manufacturing services to biopharma companies. For example, TPG acquired Indian-based SAI Life Sciences, a CDMO with expertise across the molecular life cycle, for $135 million.
We expect biopharma deal activity to remain robust in the coming year, particularly in niche services and clinical-stage pharma assets. Continued downward pressure on pricing will amplify the need for efficient drug development and commercial effectiveness. A dip in biotech public markets and a cooled IPO environment could lead more small and midsized clinical-stage drugmakers to explore other financing opportunities with corporate acquirers or buyout firms. Given the steep competition for biopharma assets from both sets of acquirers, however, the average deal value for biopharma assets keeps rising. Investors need to have a clear value-creation thesis to win the asset and have an operational plan in place if they hope to realize the expected returns.