Global Healthcare Private Equity Report
 }
}
At a Glance
- Although the life sciences sector continues to attract buyout activity, private equity deal value in 2023 struggled to match the levels of 2022.
- While the biotech funding slowdown is still a hurdle, investors began displaying more comfort putting money to work in early-stage therapies and precommercial medical devices.
- The ripple effects of the Inflation Reduction Act’s drug negotiation provisions, coupled with greater investment in cell, gene, and RNA therapies, will have broad implications for services providers.
- Demand for glucagon-like peptide-1 agonists (GLP-1s)—such as Wegovy, Ozempic, Mounjaro, and Zepbound—is boosting the need for ingredients or services that support GLP-1 manufacture.
This article is part of Bain's 2024 Global Healthcare Private Equity Report.
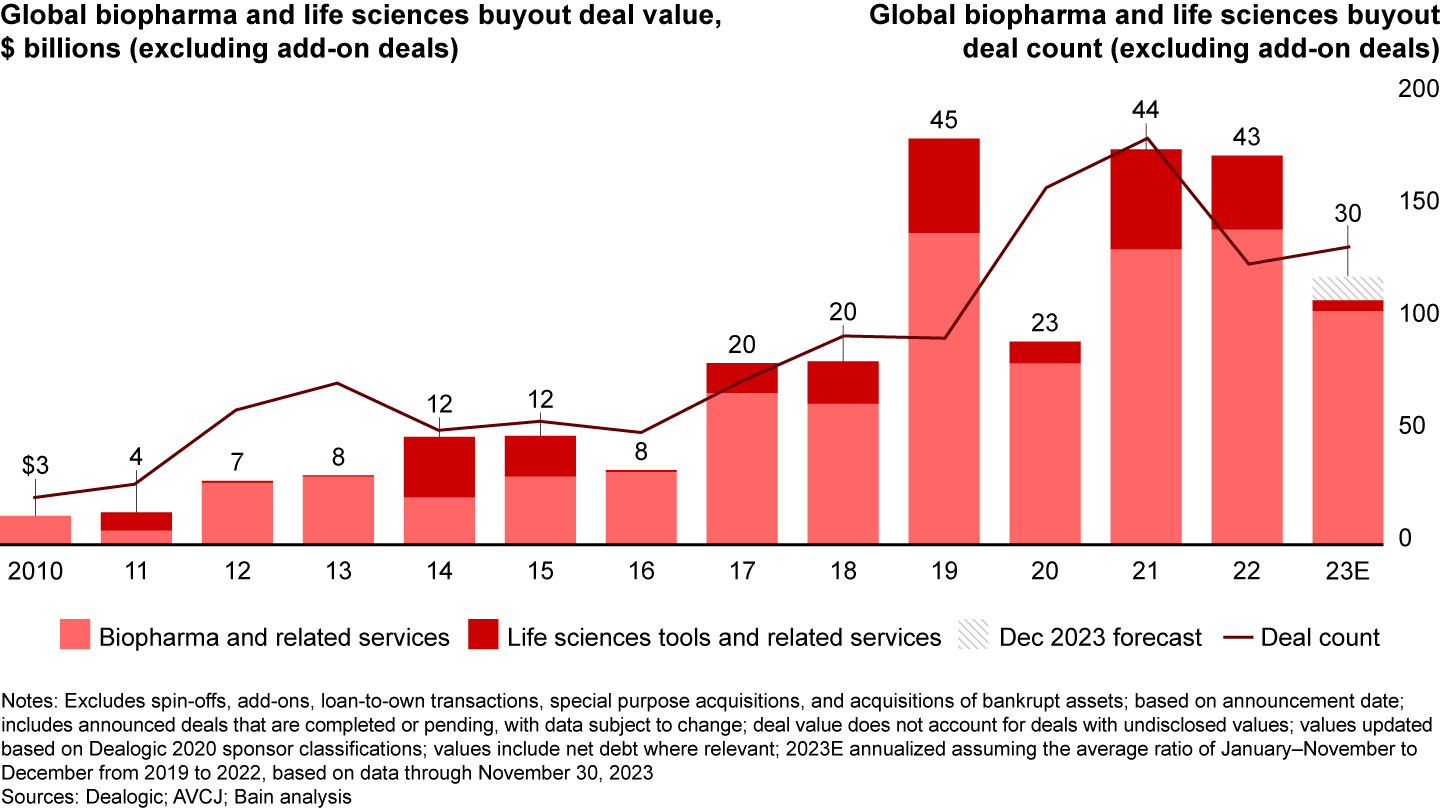
The life sciences sector has assumed a growing importance to private equity (PE) funds as investors look for innovative, recession-resistant businesses. From 2018 through 2022, PE investments into the biopharma, life-sciences tools, and diagnostics sectors surged, with approximately 650 buyout deals consummated globally. Pharma services—including contract research organizations (CROs) and contract development manufacturing organizations (CDMOs)—continued to be an active vertical within life sciences, with deal value above 2022 levels. However, in the face of economic uncertainty and continued high interest rates, overall life sciences deal value in 2023 struggled to maintain last year’s pace (see Figure 1). That said, continued success will require expertise on the part of funds, including operational and commercial excellence, in addition to scientific proficiency.

Four themes shaping life sciences investments
Four key forces are shaping the life sciences investment landscape and innovation:
- the rise of the class of medications known as glucagon-like peptide-1 agonists (GLP-1s);
- the Inflation Reduction Act’s (IRA) drug negotiation provisions and its ripple effects;
- increased investment in advanced modalities such as cell, gene, and RNA therapies and the implications for services providers; and
- growing private equity interest in making investments in early-stage therapies and precommercial medical devices.
Coupled with those forces, the rise of artificial intelligence (AI) and its ability to accelerate product development and commercialization will continue to transform life sciences (see “Generative AI Will Transform Healthcare” and “Healthcare IT Hits a Speed Bump”).
The promise and potential of GLP-1s
Over the past year, sales in the GLP-1 class of medications surged. Indeed, sales of Novo Nordisk’s semaglutide—marketed under the brand names of obesity drug Wegovy and diabetes drug Ozempic—and Eli Lilly’s tirzepatide—sold under the brand names of Mounjaro for diabetes and Zepbound for obesity—have been limited only by the ability of manufacturers to produce them. Initially used to treat Type 2 diabetes, the ability of semaglutide and tirzepatide to reduce weight and evidence of their positive effect on cardiovascular and renal health have increased the appetite for GLP-1s among prescribers and patients.
Given the potential market for these drugs, three implications confront PE investors:
- Demand for inputs or services supporting the manufacturing of GLP-1 therapies will likely increase. Firms that supply these goods—such as contract development and manufacturing organizations (CDMOs) focused on peptides and biologics and manufacturers of delivery device components such as autoinjectors—are poised to benefit from the growing demand and current supply constraints.
- An expanded ecosystem to support patients will be needed, including services to identify, qualify, and enroll eligible patients, as well as physical or digital health platforms to support patients on these therapies.
- Finally, investments with business models based on high rates of obesity could see their long-term growth projections decline. Given the novelty of GLP-1s, their ultimate effect for now is unclear. Still, investors should evaluate the implications for returns under a scenario in which obesity rates decrease materially.
Ripple effects of the Inflation Reduction Act have begun
The direct drug price negotiation provisions of the IRA could affect global biopharmaceutical companies profoundly given the outsize contribution of the US market to biopharma revenues and profits. The first round of products subject to negotiation has now been announced, and by early 2024 pharmaceutical companies will have a clearer idea of the negotiated maximum fair prices that go into effect in 2026 to participate in Medicare. PE investments in the biopharmaceutical sector have tended to focus on derivative plays such as services and supporting IT, and the impacts of drug negotiations look to be nuanced for investors.
For example, biopharmaceutical companies are expected to focus even more on biological molecules such as antibodies, given the longer period between FDA approval and price negotiation the IRA provides (13 years for biologics vs. 9 years for small molecules). Biopharmaceutical companies will seek to be strategic, conducting multiple drug trials for the same molecule in concert so as to maximize its use and application. For example, a molecule with the opportunity to be used in psoriasis and rheumatoid arthritis may undergo trials on both simultaneously so that in the event that the drug is approved for both conditions, the pharmaceutical company can get the most out of its time window before Medicare negotiations.
Understandably, this is the domain of the biopharmaceutical giants that have the financial and commercial wherewithal to coordinate multiple trials simultaneously and scale up manufacturing quickly. In that case, more biologic assets may change hands during development, from smaller biotech companies to biopharma giants. Indeed, there may be increased appetite among firms of any size to partner with capital providers to support the development of promising molecules.
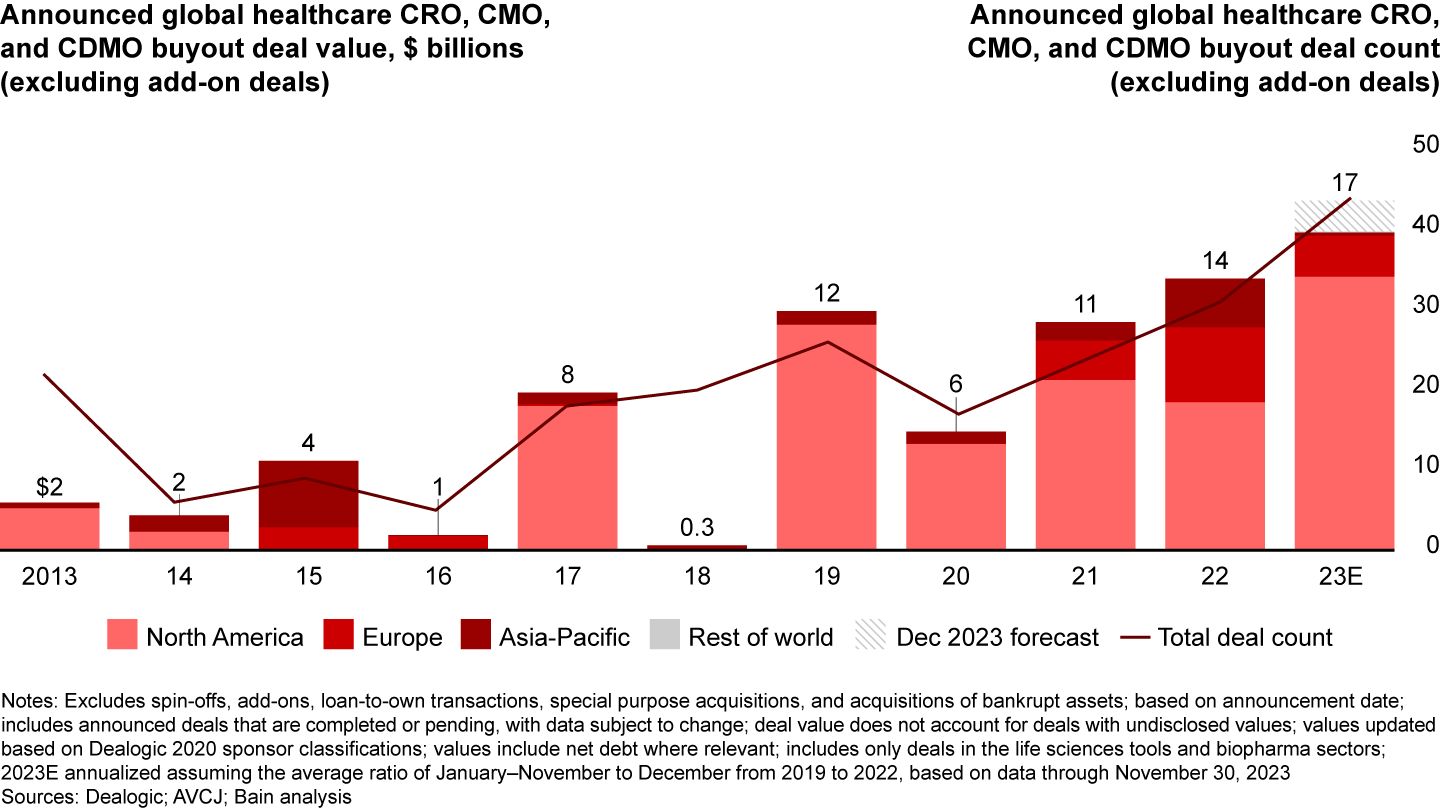
This effect, in turn, would be expected to flow through into supporting infrastructure, such as contract development and manufacturing organizations (CDMOs) (see Figure 2).

Early innings of growth in advanced modalities
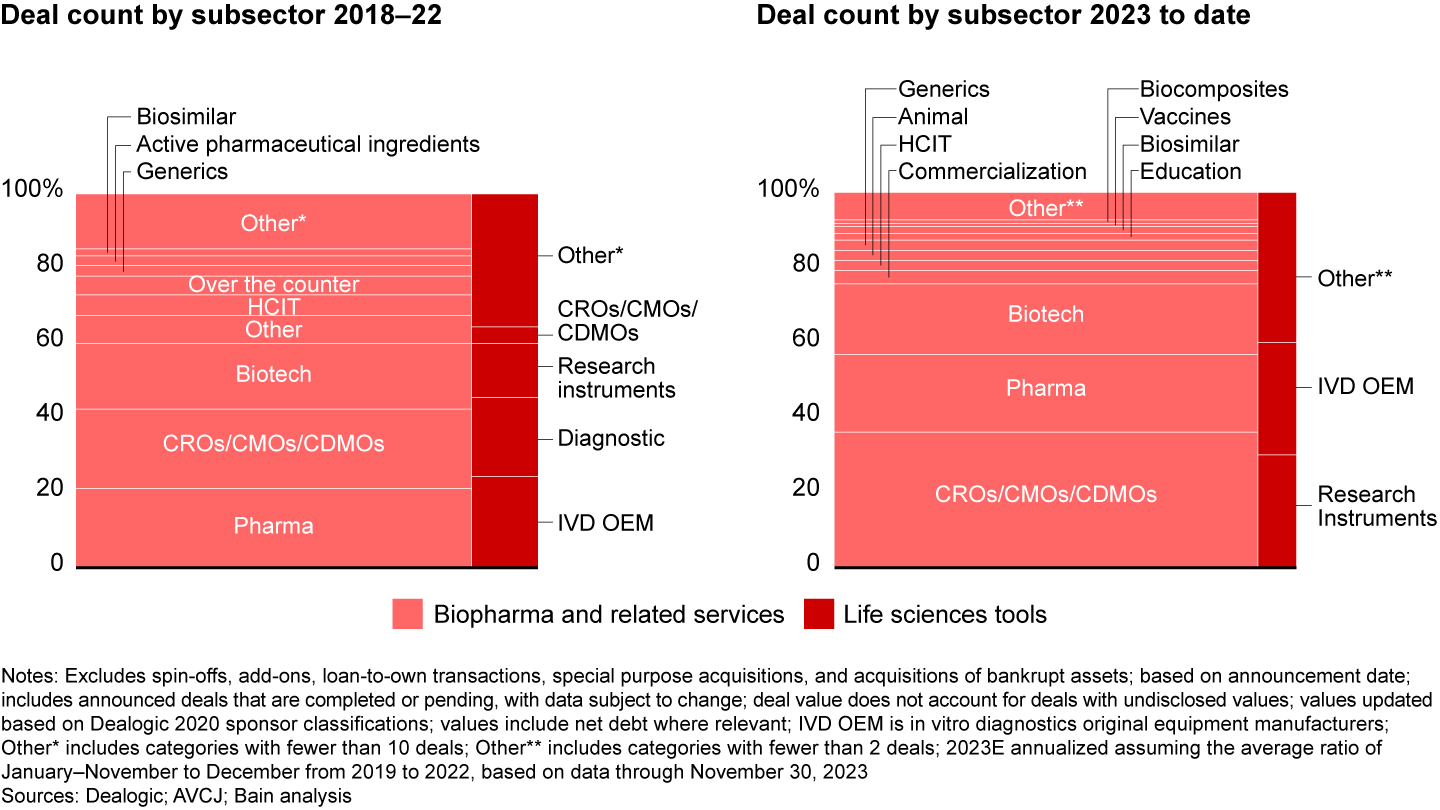
Next-generation modality therapeutics such as cell therapies, gene therapies, mRNA and other RNA therapies, and antibody drug conjugates are riding a long-term growth trend. Growth has stemmed from leaps in fundamental research as well as regulatory and commercial dynamics such as the Inflation Reduction Act. Each modality has unique discovery, development, and manufacturing processes that require discrete inputs and services to support the advancement of products.
Investment opportunities in this space are tricky to source and win given the requirement to find technical niches and invest ahead of the derisking of the modality. But for those prepared to compete here, the payoffs can be huge. This year, for example, ArchiMed and Warburg Pincus exited Polyplus in a sale to Sartorius Stedim Biotech for $2.6 billion.
The disruption brought about by innovation in advanced modalities has led big pharmaceutical companies and small independent biotechs alike to turn to outsourced service providers to complement in-house investments—witness Bayer’s $250 million investment in a Parkinson’s Disease cell therapy manufacturing facility. In turn, investors have sought to invest in contract research organizations (CROs), contract manufacturing organizations (CMOs), and CDMOs with deep ties to pharma sponsors (see Figure 3). Two of the year’s three largest deals were contract organizations—the taking private of Syneos Health and the carve-out of Baxter BioPharma Solutions (now known as Simtra Biopharma Solutions). Investors had the strongest appetite for contract organizations with specialized capabilities and expertise, such as Simtra’s strength in sterile fill/finish, and smaller preclinical-focused CROs in Europe.

PE interest in early-stage therapies and precommercial devices has grown
Historically, activity involving early-stage therapies and precommercial medical devices has been uncommon given the event-driven nature of these investments. But the evolving macro environment and deal market is making some investors increasingly comfortable with evaluating and underwriting such investments. For example, in November TPG led an $85 million series B investment into MBrace Therapeutics, a preclinical antibody-drug conjugate player, via the TPG Life Sciences Innovations Fund (TPG LSI) and The Rise Fund.
Competition with strategics for these assets raises the bar for technical and operations due diligence on the part of private equity. Additionally, it’s essential to assess value creation opportunities during due diligence to determine whether private equity ownership offers greater growth potential than strategic ownership. To do this, private investors are building expertise in many ways including upskilling technical investment teams, acquiring smaller life sciences-focused funds (such as Carlyle’s acquisition of Abingworth in 2022), raising life sciences-focused investment vehicles, and partnering more closely with professional services firms and operating experts.
Investors look past the sound and the fury
Looking ahead, life sciences innovation appears to be robust and well positioned to deliver pioneering products that solve long-standing health needs and generate economic and societal value. While the biotech sector ran into difficulties with fund-raising in 2023, investors appear to be looking past the short-term noise and are prepared to invest in promising life science innovations and in the services, products, and IT solutions to support those advances. As a result, this domain has the potential to create attractive returns for their limited partners while improving patient outcomes.